1 Chloro 3 Methyl Butane
couponhaat
Sep 23, 2025 · 7 min read
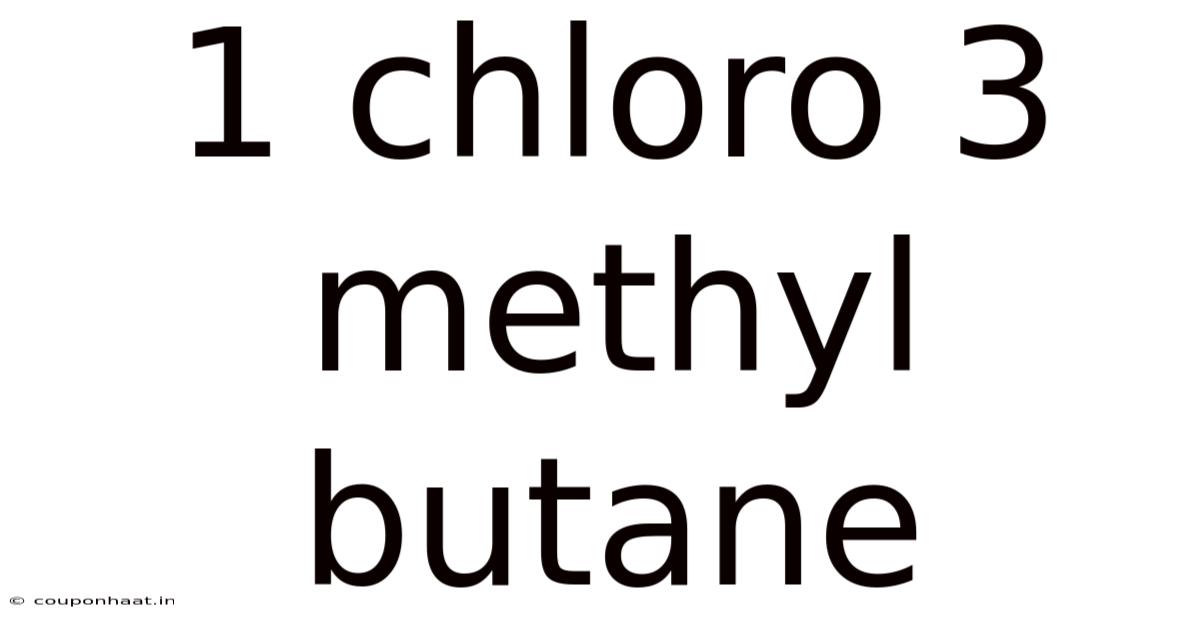
Table of Contents
Decoding 1-Chloro-3-methylbutane: Structure, Properties, and Reactions
1-Chloro-3-methylbutane, often abbreviated as 1-chloro-3-methylbutane, is an organic compound belonging to the alkyl halide family. Understanding its structure, properties, and reactivity is crucial for anyone studying organic chemistry or working with halogenated hydrocarbons. This comprehensive guide will delve into the specifics of this compound, explaining its characteristics, synthesis methods, reactions, and safety considerations. We will also explore its potential applications and environmental implications.
Introduction: Unveiling the Structure of 1-Chloro-3-methylbutane
1-Chloro-3-methylbutane's chemical formula is C₅H₁₁Cl. The "1-chloro" prefix indicates that the chlorine atom (Cl) is attached to the first carbon atom in the longest carbon chain, while "3-methyl" signifies a methyl group (CH₃) branching off from the third carbon. This nomenclature follows IUPAC (International Union of Pure and Applied Chemistry) rules for naming organic compounds. Understanding this nomenclature is critical to predicting the compound's properties and reactivity. Let's visualize its structure:
The molecule's structure can be represented in several ways, including:
- Condensed formula: CH₃CH₂CH(CH₃)CH₂Cl
- Skeletal formula: A simplified representation showing only the carbon backbone and functional groups. (Imagine a chain of five carbons, with a methyl group on the third carbon and a chlorine on the first).
- 3D model: A three-dimensional representation illustrates the spatial arrangement of atoms, including bond angles and conformations.
Physical and Chemical Properties: A Closer Look
Several key physical and chemical properties distinguish 1-chloro-3-methylbutane:
- Appearance: It's a colorless liquid at room temperature.
- Odor: It possesses a characteristic pungent, slightly sweet odor common to many halogenated hydrocarbons. Caution: Direct inhalation should be avoided due to potential toxicity.
- Boiling point: Around 106-108 °C. The boiling point is relatively higher than similar alkanes due to the presence of the polar C-Cl bond.
- Solubility: It's largely insoluble in water, a typical trait for non-polar organic compounds. However, it is soluble in many organic solvents like ether and alcohol.
- Density: Less dense than water.
- Reactivity: The presence of the C-Cl bond makes it susceptible to nucleophilic substitution and elimination reactions. This reactivity is central to many of its applications and synthetic uses. The relatively weak C-Cl bond is prone to cleavage, leading to various reactions. The primary carbon (where the chlorine is attached) is less sterically hindered than a secondary or tertiary carbon, influencing reaction rates.
Synthesis and Preparation: Crafting 1-Chloro-3-methylbutane
Several methods can be used to synthesize 1-chloro-3-methylbutane. The most common approach involves the reaction of 3-methyl-1-butanol with a chlorinating agent, such as thionyl chloride (SOCl₂) or phosphorus pentachloride (PCl₅):
-
Reaction with Thionyl Chloride (SOCl₂): 3-methyl-1-butanol reacts with SOCl₂ to produce 1-chloro-3-methylbutane, sulfur dioxide (SO₂), and hydrogen chloride (HCl). This reaction is preferred due to the gaseous byproducts, simplifying purification.
-
Reaction with Phosphorus Pentachloride (PCl₅): Similar to the above, PCl₅ also reacts with 3-methyl-1-butanol to form 1-chloro-3-methylbutane, along with phosphorus oxychloride (POCl₃) and hydrogen chloride (HCl).
The reaction mechanism for both methods involves the formation of an intermediate alkyl chlorosulfite or alkyl phosphorochloridate, which then undergoes elimination to form the final product. Optimizing reaction conditions, such as temperature and solvent selection, is crucial for maximizing yield and minimizing side products.
Other methods, though less common, might involve free radical chlorination of 3-methylbutane, but this approach will likely produce a mixture of isomers, requiring further purification steps to isolate the desired 1-chloro-3-methylbutane.
Chemical Reactions: Exploring the Reactivity of 1-Chloro-3-methylbutane
The presence of the reactive C-Cl bond makes 1-chloro-3-methylbutane a versatile reactant in various organic reactions. The key reactions include:
-
Nucleophilic Substitution (SN1 and SN2): The chlorine atom is a good leaving group, making the molecule susceptible to nucleophilic attack. The reaction mechanism can follow either SN1 (unimolecular nucleophilic substitution) or SN2 (bimolecular nucleophilic substitution) pathways, depending on the steric hindrance around the carbon bearing the chlorine and the nature of the nucleophile. Strong nucleophiles in polar aprotic solvents favor SN2, while weaker nucleophiles in polar protic solvents favor SN1.
-
Examples of Nucleophilic Substitution:
- Reaction with hydroxide ion (OH⁻): This leads to the formation of 3-methyl-1-butanol through an SN2 reaction.
- Reaction with cyanide ion (CN⁻): This forms 4-methylpentanenitrile.
- Reaction with alkoxide ions (RO⁻): Leads to the formation of ethers.
-
Elimination Reactions (E1 and E2): Under specific conditions, such as high temperatures and the presence of a strong base, 1-chloro-3-methylbutane can undergo elimination reactions, yielding alkenes. The reaction can follow either E1 (unimolecular elimination) or E2 (bimolecular elimination) pathways, depending on the reaction conditions and the strength of the base.
-
Examples of Elimination Reactions:
- Reaction with a strong base like potassium tert-butoxide (t-BuOK): This leads to the formation of 3-methyl-1-butene as a major product through an E2 mechanism. Other isomers may be formed as minor products.
-
Grignard Reaction: Although less direct, the chlorine atom can be replaced with magnesium to form a Grignard reagent, which is extremely reactive and useful in forming carbon-carbon bonds.
Applications and Uses: Exploring the Practical Significance
While not as widely used as some other alkyl halides, 1-chloro-3-methylbutane finds applications in several areas:
-
Organic Synthesis: It serves as a valuable intermediate in the synthesis of more complex organic molecules. Its reactivity allows for the introduction of various functional groups, making it a useful building block.
-
Solvent: Its solvent properties, although limited by its reactivity, may be employed in specific niche applications.
-
Research and Development: It is frequently utilized in research settings as a model compound for studying reaction mechanisms and exploring the reactivity of alkyl halides.
Safety and Environmental Considerations: Handling with Care
As with most organic halides, 1-chloro-3-methylbutane requires careful handling due to several safety and environmental concerns:
-
Toxicity: It is considered a moderately toxic compound. Inhalation, ingestion, or skin contact should be avoided. Appropriate personal protective equipment (PPE), including gloves, eye protection, and respiratory protection, should be used when handling it.
-
Flammability: It is a flammable liquid. Keep away from ignition sources and handle in a well-ventilated area.
-
Environmental Impact: Its release into the environment can be harmful to aquatic life and contribute to soil and water pollution. Proper disposal methods are necessary to minimize environmental impact.
Frequently Asked Questions (FAQ)
-
Q: What is the difference between 1-chloro-3-methylbutane and 2-chloro-3-methylbutane?
- A: The difference lies in the position of the chlorine atom. In 1-chloro-3-methylbutane, the chlorine is attached to the first carbon, while in 2-chloro-3-methylbutane, it's attached to the second carbon of the longest carbon chain. This difference significantly affects their reactivity and properties.
-
Q: Is 1-chloro-3-methylbutane chiral?
- A: No, 1-chloro-3-methylbutane is not chiral. It lacks a chiral center (a carbon atom bonded to four different groups).
-
Q: How can I safely dispose of 1-chloro-3-methylbutane?
- A: Consult your local hazardous waste disposal regulations. Do not pour it down the drain or into the environment.
-
Q: What are the main health hazards associated with 1-chloro-3-methylbutane?
- A: Inhalation can cause respiratory irritation. Skin contact can lead to skin irritation or burns. Ingestion is extremely hazardous and can cause serious health problems.
Conclusion: A Comprehensive Overview
1-chloro-3-methylbutane, although perhaps not a household name, represents a fascinating example of an alkyl halide with significant applications in organic chemistry. Understanding its structure, properties, synthesis, and reactivity is essential for anyone working with or studying organic compounds. Always remember to prioritize safety when handling this compound and adhere to proper disposal procedures to protect both human health and the environment. Further research into its applications and potential uses might reveal even greater significance in various scientific and industrial fields.
Latest Posts
Related Post
Thank you for visiting our website which covers about 1 Chloro 3 Methyl Butane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.